We are thrilled to introduce the newest member of the Notes From Nature community: Natural History at ROM Revealed!
This exciting new project invites you to explore and transcribe specimens from the Royal Ontario Museum’s vast natural history collections—collections that bring together the worlds of biology, history, and nature in extraordinary ways.
Natural history collections hold thousands of untold stories. Each specimen label captures a snapshot of life: where a species lived, when it was collected, and what the environment looked like at that moment in time. These records are priceless tools for understanding how life on Earth has changed. Yet many of them remain hidden in old handwritten or typed labels that haven’t been read in decades. That’s where you come in.
The Royal Ontario Museum (ROM), one of North America’s leading cultural institutions, is home to an incredible 18 million objects, with natural history forming a vital part of its mission. Our first set of expeditions will shine a spotlight on insects and plants from across Canada—two incredibly diverse and ecologically important groups with so many specimens still waiting to be digitized.
By helping transcribe these labels, you’re doing more than converting labels into data—you’re unlocking scientific knowledge that fuels biodiversity research around the world. Your transcriptions will be shared through Canadensys and the Global Biodiversity Information Facility (GBIF), where they become part of global conservation action, scientific discovery, environmental policy, and education. These two open-access platforms can be used worldwide by anyone with an internet connection.
Whether you’re a returning Notes From Nature volunteer or joining us for the first time, we’d love to have you along for this journey. Every label you transcribe helps reveal a piece of our planet’s history—and helps protect its future.
Jump in and explore the new project today!
Introducing new workflows from Capture the Collections
Hello friends,
We have a few updates to share from Capture the Collections.
First, we’re excited to announce several new workflows now launching:
- Our partnership with the University of Guelph continues with two new workflows focused on grasses (Poaceae) and sedges (Cyperaceae).
- We’re introducing our first workflows from the Flora of British Columbia project, featuring the heather family (Ericaceae) and the rose family (Rosaceae).
- Expedition Arctic Botany is back! These workflows highlight work completed in Summer 2025 by Molson students Tamara Chaput and Matthew De Roover, drawing from the lichen and liverwort collections.
We also have more difficult news to share. Herbarium digitization projects at the Canadian Museum of Nature are being paused, and Kim and Lyn will be stepping away from Capture the Collections. Until digitization efforts can resume, these workflows mark our final ones and will, we hope, help bridge the gap between the end of this digitization phase and what comes next. We appreciate your patience if there is a delay in hearing back from the project team or our guest moderators during this time.
It’s hard to fully express how grateful we are for the time you’ve spent with us—transcribing, discussing, and sharing your expertise on the Talk boards. Capture the Collections has never been just about mobilizing data; it’s also been about building community, and we truly hope you’ve felt that along the way.
Until next time,
Kim, Lyn, and Jennifer


- Capilano River Salmon Hatchery, Vancouver, British Columbia Photo: K.Madge 2. Carex bigelowii subsp. bigelowii, Kangiqtualuk Agguqti, Nunavut. Photo: P. Sokoloff
Bonjour, les amis
Nous avons quelques nouvelles à partager de la part de Capture the Collections.
Tout d’abord, nous sommes heureux d’annoncer le lancement de plusieurs nouveaux flux de travail :
- Notre partenariat avec l’Université de Guelph se poursuit avec deux nouveaux flux de travail consacrés aux graminées (Poaceae) et aux carex (Cyperaceae).
- Nous présentons nos tout premiers flux de travail issus du projet Flora of British Columbia, mettant en vedette la famille des bruyères (Ericaceae) et la famille des roses (Rosaceae).
- Expédition Botanique Arctique est de retour ! Ces flux de travail mettent en valeur le travail réalisé à l’été 2025 par les étudiantes et étudiants Molson Tamara Chaput et Matthew De Roover, à partir des collections de lichens et d’hépatiques.
Nous avons aussi une nouvelle plus difficile à partager. Les projets de numérisation des herbiers au Musée canadien de la nature sont mis en pause, et Kim et Lyn se retireront de Capture the Collections. Jusqu’à ce que les activités de numérisation puissent reprendre, ces flux de travail marquent nos derniers projets et aideront, nous l’espérons, à faire le lien entre la fin de cette phase de numérisation et la suite. Nous vous remercions de votre patience si un délai survient dans les réponses de l’équipe du projet ou de nos modératrices et modérateurs invités durant cette période.
Il est difficile d’exprimer pleinement à quel point nous vous sommes reconnaissants pour le temps que vous avez passé avec nous — à transcrire, à échanger et à partager vos connaissances sur les forums de discussion. Capture the Collections n’a jamais été uniquement une question de mobilisation des données ; il s’agit aussi de bâtir une communauté, et nous espérons sincèrement que vous l’avez ressentie tout au long de cette aventure.
À la prochaine,
Kim, Lyn et Jennifer
Survey Request!
Help us find out more about our volunteers! As part of a PhD research effort*, we’re exploring who volunteers on citizen science projects like Notes from Nature and why. We invite you to complete a brief survey. The questionnaire will ask about personal information such as gender, age and ethnicity. It will also ask about your experience of taking part in the project. It should take approximately 20 minutes to complete. All responses will be kept confidential and anonymized. Taking part is entirely voluntary. The survey has received ethical approval from the University of York. More information can be found via the survey link.
Thank you for your time.
*Sarah Laptain, a graduate student in the Department of Environment and Geography at the University of York, is leading this effort.
Classification for Project Cybermander is complete!
We’d like to extend our gratitude to all the contributors. We had a whopping 47,523 images classified by 1910 unique Zooniverse users as well as contributions from 2640 sessions where contributors were not logged into the Zooniverse platform.
We are very excited to dig into the data now, which will help us understand how salamanders behave when they have access to thermal differences in their environment, and how they behave with and without thermal gradients after pathogen exposure. Your contributions to Project Cybermander are helping fill gaps in this knowledge about thermoregulation habits of amphibians and will help us make disease-risk projections for these special animals.
Thank you again and we’re excited to share final results as soon as we’re finished with analyses.
— Natalie Claunch
🌱Capture the Collections × OAC Herbarium — our collaboration is sprouting new workflows!
Our collaboration with the University of Guelph continues to grow with two new and fascinating flowering plant families: the Apiaceae (carrot or parsley family) and the Orchidaceae (orchid family).
Members of the Apiaceae are easy to spot by their umbrella-like clusters of flowers. This family includes everyday favourites like carrots, celery, and parsley – as well as wild species that play important roles in ecosystems across Canada.
The Orchidaceae, in contrast, are celebrated for their beauty and fragility. Orchids grow in many parts of the world, from tropical forests to northern wetlands, and include the variety that produces the beloved vanilla bean. Their beauty and vulnerability make them an important group to study and conserve.
In these workflows, you’ll be capturing:
Collector name
Collection date
Collector number
Your transcriptions will make these specimens easier to find and use for research, education, and conservation. https://bit.ly/3rsFS0t
 Keep an eye out – we’re also preparing projects on the Flora of British Columbia and Prince Edward Island!
Keep an eye out – we’re also preparing projects on the Flora of British Columbia and Prince Edward Island!
 Capture the Collections × Herbier de l’OAC — notre collaboration fait germer de nouveaux flux de travail !
Capture the Collections × Herbier de l’OAC — notre collaboration fait germer de nouveaux flux de travail !
Notre collaboration avec l’Université de Guelph continue de croître avec deux nouvelles familles de plantes à fleurs fascinantes : les Apiacées (famille de la carotte et du persil) et les Orchidacées (famille des orchidées).
Les membres des Apiacées se reconnaissent facilement à leurs grappes de fleurs en forme de parapluie. Cette famille comprend des plantes bien connues comme la carotte, le céleri et le persil -ainsi que des espèces sauvages qui jouent des rôles importants dans les écosystèmes partout au Canada.
Les Orchidacées, quant à elles, sont célébrées pour leur beauté et leur fragilité. Les orchidées poussent dans de nombreuses régions du monde, des forêts tropicales aux milieux humides nordiques, et comprennent l’espèce qui produit la précieuse gousse de vanille. Leur beauté et leur vulnérabilité en font un groupe important à étudier et à conserver.
Dans ces flux de travail, vous transcrirez :
Le nom du collecteur
La date de collecte
Le numéro du collecteur
Vos transcriptions rendront ces spécimens plus faciles à trouver et à utiliser pour la recherche, l’enseignement et la conservation. https://bit.ly/3rsFS0t
 Restez à l’affût – nous préparons également des projets sur la flore de la Colombie-Britannique et de l’Île-du-Prince-Édouard !
Restez à l’affût – nous préparons également des projets sur la flore de la Colombie-Britannique et de l’Île-du-Prince-Édouard !
New Projects Sprouting: Draba and the Beech Family
Hello friends,
Two new workflows are sprouting up on Notes from Nature: Capture the Collections – and we think you’ll go nuts for them!
 All the Draba: Tales of Whitlow grasses
All the Draba: Tales of Whitlow grasses
Small but mighty, Draba belong to the mustard family (Brassicaceae) and thrive in cold, rocky, and windswept habitats. Sometimes called “Whitlow grasses,” these hardy wildflowers are found across North America and around the world, from Arctic tundra to high mountain slopes. Draba often grow in disturbed areas – roadsides, pastures, and gravelly soils – and are among the first plants to bloom in spring, covering the ground with tiny white or yellow flowers. Though small, Draba play a big role in helping scientists understand how plants adapt and survive in extreme environments.
 Notes from Nuts
Notes from Nuts
Now for something a little nutty: the Fagaceae – also known as the Beech family – include some very iconic trees: oaks, beeches, and chestnuts. These trees are nut-orious! They form the backbone of many forests across the Northern Hemisphere, stabilizing soils, sheltering wildlife, and producing the acorns and nuts that sustain entire ecosystems.
Wood from these trees has shaped cultures and economies for centuries – from furniture and flooring to barrels and instruments. But their scientific value is just as enduring: studying these trees helps us understand forest health, climate resilience, and long-term ecological change.
As always, your contributions make these collections searchable, shareable, and scientifically meaningful. Whether you’re here for the alpine flowers or the mighty nut – bearing trees, join us in exploring biodiversity one label at a time.
– Kim, Lyn, and Jennifer – The Capture the Collections Team


Nouveaux projets en germination : Draba et la famille des hêtres
Bonjour les ami·e·s,
Deux nouveaux flux de travail bourgeonnent sur Notes de la nature : Capturer les collections – et on pense que vous allez en devenir fous de nature!
 Tous les Draba : histoires de drabes
Tous les Draba : histoires de drabes
Petits mais puissants, les Draba font partie de la famille de la moutarde (Brassicaceae) et prospèrent dans les milieux froids, rocheux et exposés au vent. Parfois appelées « drabes » ou « whitlow-grasses », ces petites plantes robustes se retrouvent un peu partout en Amérique du Nord et ailleurs dans le monde, des toundras arctiques aux pentes montagneuses les plus élevées.
Les Draba poussent souvent dans les zones perturbées – le long des routes, dans les pâturages ou les sols graveleux – et comptent parmi les premières fleurs du printemps, recouvrant le sol de minuscules fleurs blanches ou jaunes. Malgré leur taille, elles jouent un grand rôle pour aider les scientifiques à comprendre comment les plantes s’adaptent et survivent dans des environnements extrêmes.
 Notes du noyer
Notes du noyer
Passons maintenant à quelque chose d’un peu plus casse-noisette! Les Fagaceae – aussi connues sous le nom de famille des hêtres – comprennent des arbres emblématiques comme les chênes, les hêtres et les châtaigniers. Ces arbres sont de véritables vedettes de la forêt : ils forment l’épine dorsale de nombreux écosystèmes à travers l’hémisphère Nord, stabilisent les sols, abritent la faune et produisent les glands et les noix qui nourrissent des écosystèmes entiers.
Leur bois a façonné des cultures et des économies depuis des siècles – meubles, planchers, tonneaux et instruments de musique – et leur valeur scientifique demeure tout aussi durable. Étudier ces arbres nous aide à mieux comprendre la santé des forêts, leur résilience face au climat et les changements écologiques à long terme.
Comme toujours, vos contributions rendent ces collections consultables, partageables et scientifiquement précieuses. Que vous soyez là pour les fleurs alpines ou les arbres à noix majestueux, joignez-vous à nous pour explorer la biodiversité, une étiquette à la fois.
— Kim, Lyn et Jennifer – L’équipe de Capturer les collections
WeDigBio – One week away!
WeDigBio starts in less than a week. The event takes place October 9-12, 2025. People from all over the world join together to digitize museum specimen data and to celebrate biodiversity collections. We hope you will join us at Notes from Nature.
Notes from Nature will have lots of great expeditions to contribute to. We’ll be featuring California plants, bees, environmental archaeology specimens and more, so please stop by. It’s also very helpful if you can spread the word about this unique and important event.
— The Notes from Nature Team
Introducing ArchaeoZooArchive – An Archaeological Natural History Archive!
The Florida Museum Environmental Archaeology Program is excited to bring a new adventure to all you Notes from Nature fans … the ArchaeoZooArchive … the first archaeological natural history transcribing project in Notes from Nature!
When we ask you, the wonderful community scientists, for help transcribing nature from natural history collections, we are typically talking about data from plant and animal specimens that have been collected over the past 100 years or so – maybe even as far back as 200 years! These records provide excellent insight to our modern biodiversity and environments and the data you transcribe is vital for understanding how we should cherish and manage our natural world. But you might be interested to know that all that modern specimen data provides only a TINY glimpse of a very long history of people’s relationships with nature. When you consider that people have been admiring, using, managing, and impacting natural resources for over a million years, you realize that 200 years is but the blink of an eye. So we bring you an opportunity to help scientists look deeper into time …
Here at the Florida Museum of Natural History, the Environmental Archaeology Program is devoted to the study of the deep history of human-environment interactions using the remains of plants, animals, and landscapes from archaeological sites. The Environmental Archaeology collections include literally millions of specimens from more than 800 archaeological sites representing over 10,000 years of prehistory across the circum-Caribbean (the region that includes the SE USA, Central America, Caribbean, and northern South America). With assistance from the National Science Foundation (NSF Grant DBI #1929448) we have been working hard to make these specimens more accessible to scientists and the community through improvements in curation, archival documentation, and of course, making the data about these specimens available through both specialized and open data publishers. The ArchaeoZooArchive is one step in the process – inviting the community to help us turn our data records about animal remains from archaeological sites into usable data, one mysterious specimen card at a time!



The data collected from the EAP specimens has informed us about the longer history of how environments have impacted people and how, in turn, people have impacted their environments. But surprisingly little of our data has been fully digitized, so although much has already been learned, by making our data available more broadly, we can learn even more valuable lessons relevant to today’s major issues by documenting the millennia of “human experiments” of the past – learning from past successes and mistakes.
Those of you who have been actively engaged with community science know this part of the story all too well – alongside the vast EAP collections are the MILLIONS of associated data records – as accession folders, card cabinets, and notebooks …



Over the years we have made great strides in scanning our primarily paper records into digital formats that can be more easily shared.

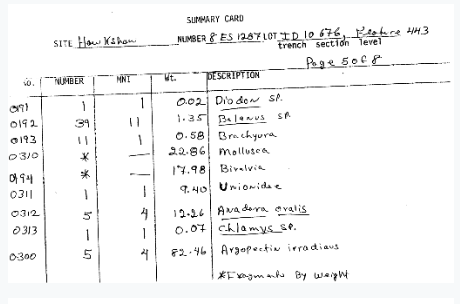
But even with digital versions, it is an archaeological project of its own to dig through these and organize, standardize, and make available the actual specimen-by-specimen data in these records.
This is where Notes from Nature has provided a wonderful opportunity to reach out to community scientists and volunteers to help us digitize the data from our scanned paper records. In collaboration with our lab team of students, researchers, and volunteers, and with the special assistance of a dedicated teen volunteer from a local high school (stay tuned to hear more from them in our next blog post), and of course with Mike Denslow of Notes from Nature, we have created a first expedition to digitize our most straight-forward data records, the Environmental Archaeology zooarchaeological specimen data summary cards! We started with zooarchaeology because it has historically been the primary focus of our collections and thus is the collection with the most standardized data recording methods and broadly accepted taxonomies in our archives. But don’t let this fool you – it will still be a challenge that we hope you’ll be excited to take on!
Today we launched ArchaeoZooArchive expeditions by geographic region, starting with South American collections! Once those have been completed we will move on to Central American and Caribbean expeditions.
Please give ArchaeoZooArchive: The South American Expedition
a try today and let us know what you think.
— Kitty F. Emery, Jessica Nickles King, Nicole Fuller, and Al Keller.
EAP logo by Al Keller. Other images by EAP.
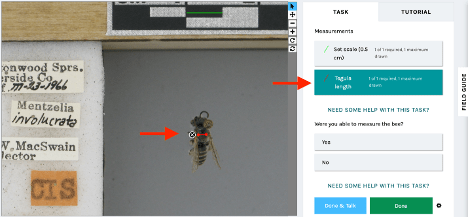
Measuring bees for Climate Change: California Edition
We are excited to launch a new measurement expedition, Measuring bees for Climate Change: California Edition. This project brings together specimen images from six institutions across the US, featuring California bees from the past 100 years. These measurements will contribute to a new study aimed at answering the question: is climate change shrinking bees?
Recently, scientists have documented body size declines in animal groups over the past 50-100 years. For insects, the causes of this shrinking effect are strongly debated. One possibility is warming temperatures interfering with normal animal development. Climate change may also affect size by restricting food availability in some environments. Regardless of cause, changes in body size can have major ecological consequences. Smaller bees, for example, carry less pollen and may be more sensitive to environmental disturbances, restricting their ability to deliver critical pollination services.


California is home to approximately 1,600 bee species, thanks to its diverse range of habitats, which include deserts, forests, and grasslands. California’s climate is warming and becoming more unpredictable, with complex and unknown impacts on bees. Notes from Nature volunteers can help us understand body size changes in California’s bees by capturing critical measurement data on historical specimens . You’ll help us measure bee body width (or intertegular distance–essentially, shoulder width), which provides a good estimate of overall body size.
Thanks to existing Notes from Nature volunteer effort, we were able to show the value of citizen community science approaches to bee measurements. In a recent paper we found that Notes from Nature volunteers provide highly accurate bee body size measurements, differing only 2% from trained researchers (link to study). This study showed the enormous potential for community scientists to unlock the wealth of size data stored in museum records.
Our preliminary results hint at exciting potential links between bee size and climate–more measurements will help fill in some critical gaps that we haven’t been able to cover yet. We need help completing these measurements to expand our dataset to represent a greater diversity of bees over time and in different habitats. This data will help us answer questions like: are bees really shrinking? Are males and females shrinking at different rates? Do hotter regions cause greater shrinking? Which habitats are most vulnerable to these effects? Questions like this require a huge amount of data and have not yet been answered for bees at this scale. Community science measurements will help us overcome this data challenge and provide new insights into bee biology in a warming world.
Please visit Notes from Nature – Big Bee Bonanza! today to help us complete the Measuring bees for Climate Change: California Edition expedition.
Notes from Nature: Capture the Collections updates
Hello friends,
Notes from Nature: Capture the Collections is thrilled to share some updates with you!
Maple Nation: Acers of Canada
We’ve just launched a brand-new workflow focused on Canada’s iconic maple trees (Acer). From the Sugar Maple (Acer saccharum) behind maple syrup to the towering Bigleaf Maple (Acer macrophyllum) spreading its canopy along the Pacific coast, these trees are central to our landscapes, culture, and history. In this workflow, you’ll be helping us transcribe collector information, locality details, and coordinate values.
We continue to love the OAC Herbarium!
Thanks to your help, the fern workflow at the University of Guelph’s OAC Herbarium was a great success! The project now continues with another exciting group: the Asteraceae, also known as the daisy or sunflower family. Among the most diverse plant families worldwide, they occur in nearly every Canadian ecosystem, including the Artic, to the Rockies, boreal forests, prairies and coastal sand dunes. They have plenty of stories to tell, and your transcriptions will help bring those stories to light. In this workflow, you’ll be capturing collector details (name, date, collector number) to make these specimens more accessible.
As always, the label data you capture supports scientists, historians, educators, and conservationists. Your contributions make these collections searchable, shareable, and meaningful for people everywhere.
Thank you for being part of Capture the Collections—we couldn’t do this without you! And don’t forget to share your discoveries on the Talk boards.
Kim, Lyn and Jennifer
The Capture the Collections team
Start transcribing on Zooniverse
Learn more about the project here
Bonjour les amis
Notes de la nature – Capturer des collections est ravis de partager quelques nouveautés avec vous!
La nation de l’érable : Acers du Canada
Nous venons de lancer un tout nouveau flux de travail consacré aux emblématiques érables du Canada (Acer). De l’Érable à sucre (Acer saccharum), qui nous donne le sirop d’érable, à l’imposant Érable à grandes feuilles (Acer macrophyllum), qui étend sa canopée le long de la côte Pacifique, ces arbres occupent une place centrale dans nos paysages, notre culture et notre histoire. Dans ce flux de travail, vous nous aiderez à transcrire les informations sur les collecteurs, les données de localité et les coordonnées.
Nous continuons d’adorer l’herbier OAC!
Grâce à votre aide, le flux de travail sur les fougères de l’herbier OAC de l’Université de Guelph a été un grand succès! Le projet se poursuit maintenant avec un autre groupe passionnant : les Asteraceae, aussi appelées la famille des marguerites ou des tournesols. Parmi les familles de plantes les plus diversifiées au monde, on peut les retrouver dans quasi tous les habitats et écosystèmes canadiens, incluant l’Arctique, les Rocheuses, la foret boréale, les praires et les dunes côtières. eElles ont beaucoup d’histoires à raconter -et vos transcriptions contribueront à les mettre en lumière. Dans ce flux de travail, vous saisirez les détails sur les collecteurs (nom, date, numéro du collecteur) afin de rendre ces spécimens plus accessibles.
Comme toujours, les données d’étiquettes que vous capturez soutiennent les scientifiques, les historiens, les éducateurs et les spécialistes de la conservation. Vos contributions rendent ces collections consultables, partageables et utiles pour tous.
Merci de faire partie de Capture the Collections — nous ne pourrions pas y arriver sans vous! Et n’oubliez pas de partager vos découvertes sur les forums de discussion.
Kim, Lyn et Jennifer
L’équipe Capture the Collections